Scientific concept – summary
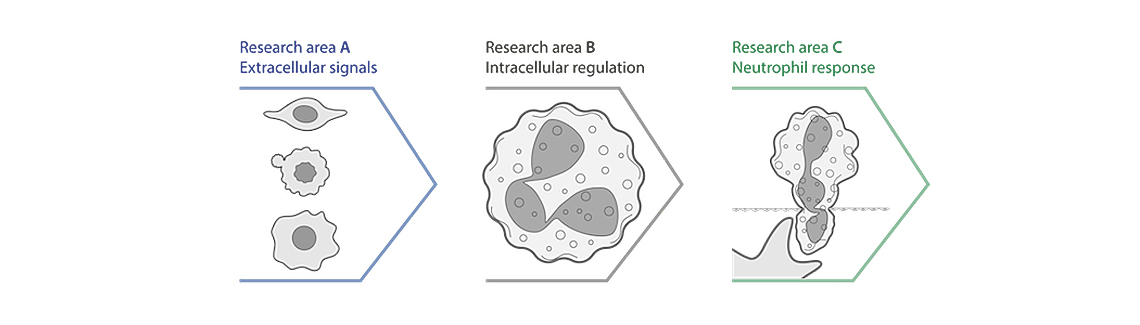
Neutrophils, the most abundant circulating white blood cells in humans, have traditionally been considered a homogenous population of terminally differentiated cells with a well-defined and highly conserved function. Indeed, their short lifespan, their inability to proliferate, their limited capacity of de novo gene expression, and their limited ability to recirculate from the tissue to the blood-stream have for long sustained this idea. However, evidence accumulated over the past ten years has demonstrated a thus far underestimated functional versatility and phenotypic heterogeneity of the neutrophil population. Far beyond their antimicrobial functions, neutrophils are emerging as decision-shapers during chronic inflammation, tumour development, but also during homeostasis. This Collaborative Research Centre Transregio (CRC TRR) 332 brings together experts from three applicant universities, the WWU Münster, the LMU Munich, and the University Duisburg-Essen as well as two associated institutions, namely the TU Dresden and the ISAS Leibniz Institute Dortmund, to decipher timely issues on neutrophil biology. Our studies in project area A dissect how environmental signals shape neutrophil production, phenotype, and function.
Here, our efforts will cover the importance of diverse microenvironmental cues including tumour niches, metabolic alterations, and inflammatory settings. In project area B we will study how intracellular processing of signals regulates neutrophil function. Specifically, we cover aspects of the rearrangement of the actin cytoskeleton, the importance of nuclear receptors and transcription factors for gene expression, as well as the ability of neutrophils and their progenitors to be trained. Functional output of neutrophils including their recruitment to tissues, their extrusion of neutrophil extracellular traps (NETs) and their death and subsequent clearance is of primary importance for therapeutic interference. Hence, projects defined in project area C will not just re-veal a refined understanding of neutrophil function but also provide a translational link with assessment of preclinical interference strategies, some of which will be consolidated by the integration of analyses of patient samples. Our efforts are complemented with a central platform providing state of the art in situ mass spectrometry imaging and multiplex immunohistochemistry to visualize neutrophils in context. Finally, an informatics platform offers a tailored infrastructure for data management, analysis, visualisation, and public outreach.